Gcp Training Courses Nih Stroke
SCDM 2. 01. 7 Annual Conference Orlando, FL, September 2. Clinical trial programs have grown in complexity, scope and scale. Electronic systems have enabled fewer people to manage larger and larger scale projects with higher quality. Course+Objectives+Discuss+the+purpose+and+various+sponsors+of+clinical+research..jpg' alt='Gcp Training Courses Nih Stroke' title='Gcp Training Courses Nih Stroke' />Further, with the advent of electronic data collection and submissions, regulators are raising the bar for what is expected for clinical trial data to be accepted as credible, corroborated, and consistent. Paper models and processes converted to electronic are no longer sufficient and the landscape is changing swiftly. Discover from those have been there and done that Hopkins University uses 3 monitors to manage over 2. CT Scans. A global pharmaceutical company has a sequence of VAS scales randomly assigned to Phase 1 patients, timed and controlled by the site coordinator. But animals are patients with data to manage also. Why+Monitor+Safety+Not+an+IND+study+Funded+through+NIH.jpg' alt='Gcp Training Courses Nih Stroke' title='Gcp Training Courses Nih Stroke' /> Atlanta Zoo maintains a global registry of all great apes and tracks their heart disease with more than 6. Another pharmaceutical company has animal health projects that includes identification of patients via RFID, calculates wound area with tracing a photograph on a tablet, patient diaries are completed via the internet. NIHs Exclusive Licenses to Biotech, Pharma StartUps Lots of Secrecy, Few Successes Its wellknown that the National. Attend the largest international, multidisciplinary educational event for clinical data managers and related professionalsJoin the NASDAQ Community today and get free, instant access to portfolios, stock ratings, realtime alerts, and more Join Today. Greg Paul was born in England. By the age of 24, he was the youngest senior quantity surveyor in the country at a wellrespected leading national house builder. Newsletter with articles on current research finds, industry news, job listings and event schedules. Includes sign up form and information about the company. Download Jumpstart Wifi. Discover the educational and social program for the SCDM 2017 Annual Conference. Learning objectives, speakers, chairs and description of the sessions. Aratana pharmaceuticals will share their perspective and lessons learned through carrying out multiple studies using EDC in multiple sites. Integration of disparate data sources makes the data management role more complex and gives rise to platforms where data managers must learn and support a wide range of studies, study types, and build types from DIY to full service and supports greater consistency, efficiency, and reuse as studies progress between phases and complexity with emerging technology. Though this panels collective experiences, you will gain insight to keep up with the evolving landscape for data collection processes and procedures. Speakers will present case studies and describe the issues they encountered and how they collaborated to resolve them obtaining cleaner data faster with higher quality with the goal of submitting results sooner to regulatory agencies for approval and fewer queries about that data. Gcp Training Courses Nih Stroke Protocol
Atlanta Zoo maintains a global registry of all great apes and tracks their heart disease with more than 6. Another pharmaceutical company has animal health projects that includes identification of patients via RFID, calculates wound area with tracing a photograph on a tablet, patient diaries are completed via the internet. NIHs Exclusive Licenses to Biotech, Pharma StartUps Lots of Secrecy, Few Successes Its wellknown that the National. Attend the largest international, multidisciplinary educational event for clinical data managers and related professionalsJoin the NASDAQ Community today and get free, instant access to portfolios, stock ratings, realtime alerts, and more Join Today. Greg Paul was born in England. By the age of 24, he was the youngest senior quantity surveyor in the country at a wellrespected leading national house builder. Newsletter with articles on current research finds, industry news, job listings and event schedules. Includes sign up form and information about the company. Download Jumpstart Wifi. Discover the educational and social program for the SCDM 2017 Annual Conference. Learning objectives, speakers, chairs and description of the sessions. Aratana pharmaceuticals will share their perspective and lessons learned through carrying out multiple studies using EDC in multiple sites. Integration of disparate data sources makes the data management role more complex and gives rise to platforms where data managers must learn and support a wide range of studies, study types, and build types from DIY to full service and supports greater consistency, efficiency, and reuse as studies progress between phases and complexity with emerging technology. Though this panels collective experiences, you will gain insight to keep up with the evolving landscape for data collection processes and procedures. Speakers will present case studies and describe the issues they encountered and how they collaborated to resolve them obtaining cleaner data faster with higher quality with the goal of submitting results sooner to regulatory agencies for approval and fewer queries about that data. Gcp Training Courses Nih Stroke Protocol
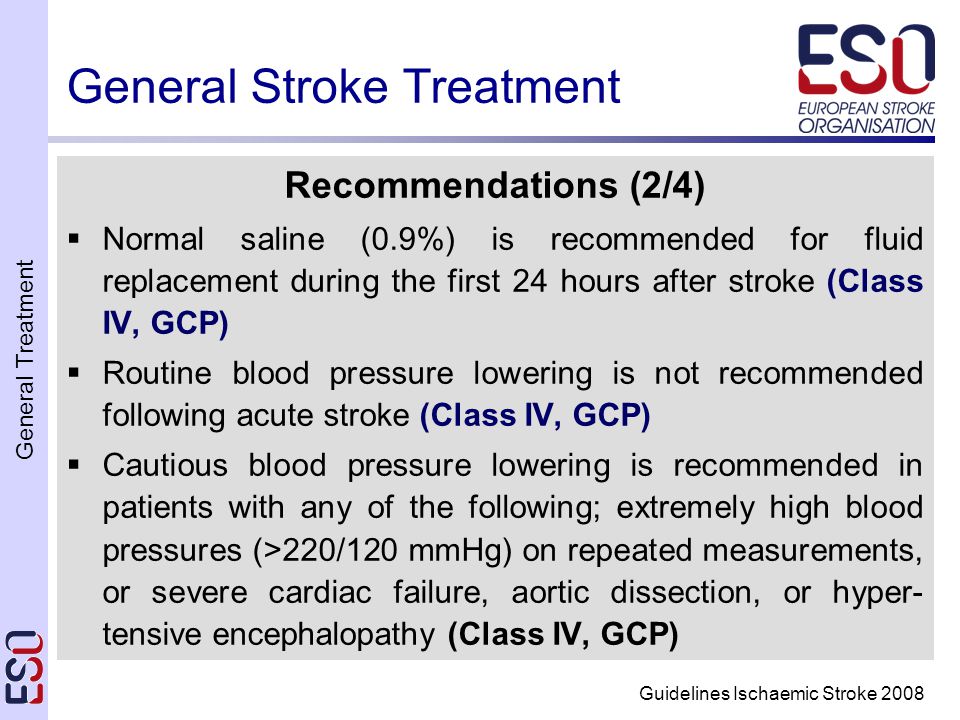 Session Chair H Karina Loyo, Ph. D., M. B. A., e. Clinical Specialist, Prelude Dynamics LLCSpeakers The Changing Role of the Data Manager in an Electronic Environment Alicia Browner, Ph. D., Chief Technology Officer, Prelude Dynamics LLCInternational Hemorrhagic Stroke Studies Optimizing EDC Systems to Facilitate Your Study Karen Lane, CCRP, Administrative Director and Research Project Manager, Department of Neurologys Brain Injury Outcomes Division, John Hopkins University. One Sponsors Perspective on the Advantages of Using EDC to Improve Study Efficiency Benefits for Data Management Jessica Wofford, DVM, Ph. D, Project Manager, Drug Development, Aratana Therapeutics, Inc. Learning Objectives Learn about the changing role of the data manager and how they might best contribute to clinical research when EDCCTMS is used. As a result of this, they will be able to identify several ways in which they can use their current skills to manage, design andor provide feedback to the electronic study build process for both do it yourself and full service study build solutions. Identify additional skills necessary to take advantage of the changing data management role in an EDCCTMS Clinical Study environment. They will be able to guide the design and edit checks of the data at the front end, ensuring cleaner data, rather than identifying and addressing problems at the back end. Learn about the capabilities of EDCCTMS from real case studies so they can include similar functionality in future clinical studies as appropriate for the research. Specifically, attendees will learn about strategies to improve and maintain interrater reliability, reduce repeated measure bias, and take advantage of EDC integrated technologies. Learn about the expansion of the data manager role, changing their perspective on EDCCTMS, and how these systems can be used to leverage small teams to manage large scale projects. Session Level Intermediate Assumes comfort within CDM industry 1 3 years experience. Certification Competencies Electronic Data Collection, Project Management, Clinical Database Design, Clinical Database Design, Clinical Trials Processes, Roles, and Responsibilities. Target Audience Study Data Manager, Study Manager, Study Clinical Director. REd. I Faculty SBIA Events. Richard Rik Lostritto, Ph. D, joined the FDA in 1. Acting Deputy Office Director for Science Policy and Acting Biopharmaceutics Lead in the Office of New Drug Quality Assessment ONDQA. Previously, Rik served as Director, Division I which includes CMC chemistry, manufacturing, and controls responsibility for oncology, hematology, cardio renal, neurology, and psychiatric drug products. He had also served as CMC Team Leader successively collocated in the Divisions of Oncology Drug Products and Pulmonary and Allergy Drug Products. Before joining the Agency, Dr. Lostritto worked at Boehringer Ingelheim Pharmaceuticals leading a group which developed medical aerosol formulations after serving as Assistant Associate Professor of Pharmacy at The University of Connecticut 1. He received his M. S. and Ph. D Degrees in Pharmaceutical Chemistry and Pharmaceutics from the University of Michigan and his BS Degree in Pharmacy from The University of Connecticut.
Session Chair H Karina Loyo, Ph. D., M. B. A., e. Clinical Specialist, Prelude Dynamics LLCSpeakers The Changing Role of the Data Manager in an Electronic Environment Alicia Browner, Ph. D., Chief Technology Officer, Prelude Dynamics LLCInternational Hemorrhagic Stroke Studies Optimizing EDC Systems to Facilitate Your Study Karen Lane, CCRP, Administrative Director and Research Project Manager, Department of Neurologys Brain Injury Outcomes Division, John Hopkins University. One Sponsors Perspective on the Advantages of Using EDC to Improve Study Efficiency Benefits for Data Management Jessica Wofford, DVM, Ph. D, Project Manager, Drug Development, Aratana Therapeutics, Inc. Learning Objectives Learn about the changing role of the data manager and how they might best contribute to clinical research when EDCCTMS is used. As a result of this, they will be able to identify several ways in which they can use their current skills to manage, design andor provide feedback to the electronic study build process for both do it yourself and full service study build solutions. Identify additional skills necessary to take advantage of the changing data management role in an EDCCTMS Clinical Study environment. They will be able to guide the design and edit checks of the data at the front end, ensuring cleaner data, rather than identifying and addressing problems at the back end. Learn about the capabilities of EDCCTMS from real case studies so they can include similar functionality in future clinical studies as appropriate for the research. Specifically, attendees will learn about strategies to improve and maintain interrater reliability, reduce repeated measure bias, and take advantage of EDC integrated technologies. Learn about the expansion of the data manager role, changing their perspective on EDCCTMS, and how these systems can be used to leverage small teams to manage large scale projects. Session Level Intermediate Assumes comfort within CDM industry 1 3 years experience. Certification Competencies Electronic Data Collection, Project Management, Clinical Database Design, Clinical Database Design, Clinical Trials Processes, Roles, and Responsibilities. Target Audience Study Data Manager, Study Manager, Study Clinical Director. REd. I Faculty SBIA Events. Richard Rik Lostritto, Ph. D, joined the FDA in 1. Acting Deputy Office Director for Science Policy and Acting Biopharmaceutics Lead in the Office of New Drug Quality Assessment ONDQA. Previously, Rik served as Director, Division I which includes CMC chemistry, manufacturing, and controls responsibility for oncology, hematology, cardio renal, neurology, and psychiatric drug products. He had also served as CMC Team Leader successively collocated in the Divisions of Oncology Drug Products and Pulmonary and Allergy Drug Products. Before joining the Agency, Dr. Lostritto worked at Boehringer Ingelheim Pharmaceuticals leading a group which developed medical aerosol formulations after serving as Assistant Associate Professor of Pharmacy at The University of Connecticut 1. He received his M. S. and Ph. D Degrees in Pharmaceutical Chemistry and Pharmaceutics from the University of Michigan and his BS Degree in Pharmacy from The University of Connecticut.